


Up: Imperfect Gases and Liquids.
Previous: Quiz
- 3.
- Write formula (9) as
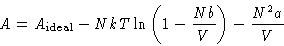
From this equation calculate the difference between the entropy of
van der Waals gas and ideal gas. What is the energy of van der Waals
gas? Explain the result.
© 1997
Boris Veytsman
and Michael Kotelyanskii
Thu Sep 18 22:50:29 EDT 1997
![]()
![]()